
Green Minute News:
Vulture populations in India have not shown signs of recovery so far since the Centre banned diclofenac drug for veterinary usage in 2006. Although this ban significantly helped reduce veterinary diclofenac use in India but pharma companies are still illegally marketing them.
Excepting Assam, in other states, toxic veterinary drugs are still being sold openly which has caused catastrophic declines in vulture populations in India.
In India, Assam has the lowest incidence of toxic veterinary drugs from the data collected, and with additional checks, it may soon be declared safe enough to initiate releases. However, other parts of India still have a lot of toxic veterinary drugs being sold.

UNDERCOVER SURVEYS
Recent undercover surveys have shown that Indian pharma companies are illegally marketing large vials which have been banned since 2015 ( as the Centre restricted the sale of human diclofenac to single-dose vials only in 2015). It is still very unclear whether this is being rigorously followed up by the Indian authorities or not.
The survey of pharmacies across India, and south Asia, carried out by local conservation teams, including many Saving Asia’s Vulture from Extinction (SAVE) partners and supported by the Royal Society for Birds (RSPB), have shown that despite nationwide bans, toxic veterinary drugs responsible for catastrophic vulture declines were still available for purchase in India.
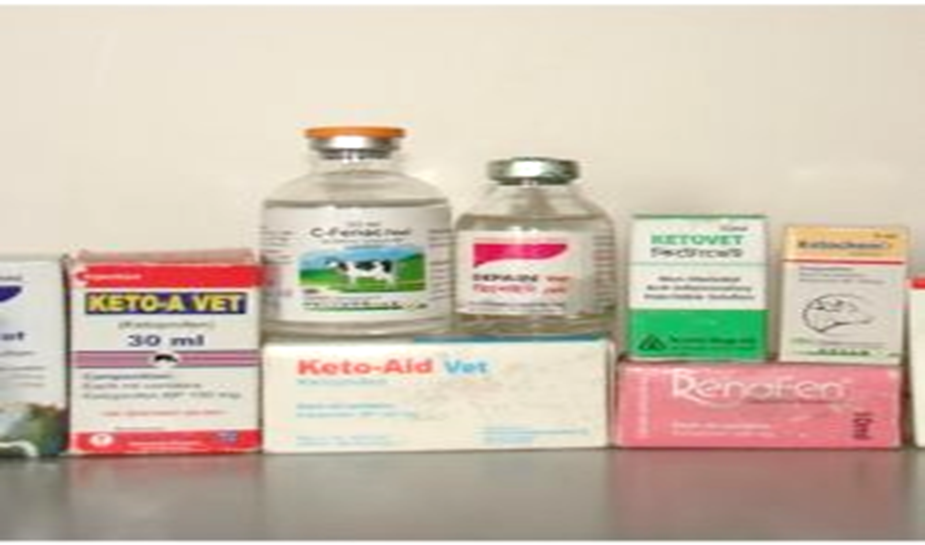
Apart from this, other drugs known, or suspected to be, toxic to vultures are still available, posing a potential long-term risk to the recovery of the vulture populations.
Recent bans by the Indian governments on toxic drugs – aceclofenac, ketoprofen, nimesulide are welcome but these drugs are still in widespread usage and that probably explains why vulture populations in India have not shown signs of recovery so far.
Members of local conservation bodies conducted undercover surveys in pharmacies across India, and the staff purchased whichever drug was offered by the pharmacist with surveys running for over 10 years.

The results revealed that not only was diclofenac still available in many regions of India, but it was still being illegally manufactured in veterinary-sized doses.
In total, undercover surveyors were sold 14 different NSAIDs, several of which have known or suspected vulture toxicity. Also, diclofenac is still available for human usage and is frequently illegally supplied for veterinary use. Several other vulture-toxic NSAIDs, such as flunixin, are still legally available, potentially hindering species recovery.

RESEARCH STUDY ON TOXIC DRUG THREAT TO VULTURES
A research paper “The continued threat of toxic NSAIDs to critically endangered Gyps Vulture in South Asia” was published in September 2025 highlights this issue in detail. John Mallord, RSPB Senior Conservation Scientist, lead author of the research paper said, “This study clearly demonstrates targeted conservation action is effective at reducing the veterinary drug threat and helping vulture populations recover. Governments in vulture-range countries, including India, can show the way by implementing the Convention for Migratory Species (CMS) Resolution, which calls for the safety-testing of all NSAIDs currently on the market, and not licensing new drugs until they have been proven safe to vultures.”
Mallord adds, “Without this obligation being fulfilled we risk jeopardising the progress made over the past 20 years in preventing the extinction of vultures in India & Asia, and to ensure the continued recovery of these amazing birds which are so vital to wildlife and humans.”

Sachin Ranade, Bombay Natural History Society, co-author of the paper said, “Our study highlights the importance of the work we do to ensure the safety of Vulture Safe Zones, especially the advocacy and education targeting local stakeholders such as pharmacists, encouraging them to stop supplying vulture-toxic drugs and to switch to safe alternatives.”
ENDEMIC VULTURE SPECIES DRIVEN TO EXTINCTION
Three vulture species that are endemic to South and South-east Asia – White-rumped, Slender-billed and Indian Vultures were driven to near-extinction due to unintentional poisoning by the veterinary drug NSAID that is diclofenac.

Vultures ingested the drug when consuming the carcasses of livestock that had been treated with diclofenac shortly before death. This resulted in kidney failure and ultimately death in millions of vultures. As a result, the veterinary use of diclofenac was banned across India in 2006.
Vultures provide an irreplaceable waste disposal service by removing animal carcasses, thereby, preventing the spread of disease. In their absence, feral dog populations have increased in some areas, leading to more cases of rabies, a significant threat to human health.
(PHOTO CREDIT: IMAGES 2,3 & 4 COURTESY SAVE WITH COLLABORATION OF WESCA WILDLIFE NETWORK, REST OF THEM – KARNATAKA FOREST DEPTT)
